Therapeutic Targets
DJ-1 Protein
Loss of and reduced function of the DJ-1 protein has been linked to the onset of a variety of diseases such as Parkinson’s disease, Alzheimer’s disease, stroke, amyotrophic lateral sclerosis, chronic obstructive pulmonary disease and type II diabetes, while DJ-1 overexpression is a marker of a variety of cancers. The DJ-1 protein is considered to be one of the primary therapeutic targets for Parkinson’s disease, as it is genetically linked to the onset of familial Parkinson’s disease and loss of its functions is associated with triggering sporadic Parkinson’s disease. DJ-1 is proposed to have multiple functions, including REDOX-sensitive antioxidant and chaperon activities. For example, it inhibits the aggregation of a-synuclein, a process linked to the onset of Parkinson’s Disease. The down-regulation of endogenous DJ-1 sensitizes neurons to cell death induced by oxidative insults, whereas elevated levels of wild-type, but not mutant, DJ-1 protect neurons from oxidative stress and other protein misfolding related insults. DJ-1 loss-of-function flies are also hypersensitive to oxidative stress and DJ-1 knockout mice show nigrostriatal dopaminergic deficits and are hypersensitive to the toxic effects of oxidative stress. Under oxidizing conditions, DJ-1 can undergo superfluous oxidation of several Cys and Met residues, which may lead to DJ-1 inactivity as a result of structural destabilization and aggregation of the protein. Over-oxidized DJ-1 has been observed in patients with the sporadic form of PD.
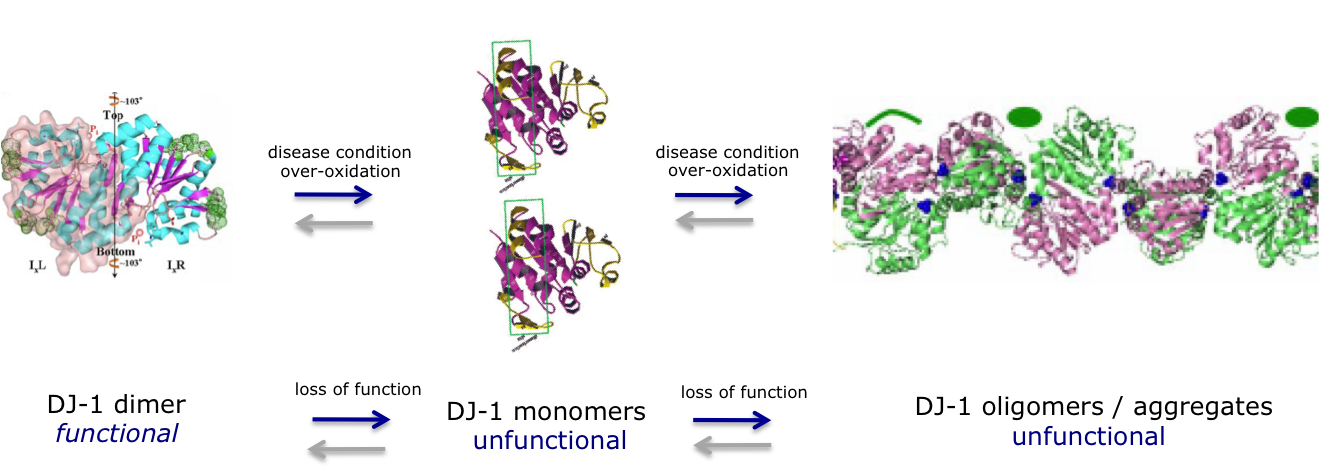
Superfluous oxidation of DJ-1 during disease conditions leads to DJ-1 inactivity as a result of structural destabilization and aggregation of the protein.
Tau protein
Aggregation of the Tau protein is linked to the onset and progression of Tauopathies, which represent a subset of neurodegenerative disorders, including Alzheimer’s disease (AD), which represents around 90% of incidences, and frontotemporal dementias (FTD). In these diseases Tau protein accumulates into protein deposits known as neurofibrillary tangles (NFTs) in the patient’s brain. There is an established link between familial mutations in the Tau gene on chromosome 17 (FTDP-17) and early-onset forms of FTD with Parkinsonism, and Tau is one of the currently most recognized targets for the potential treatment of AD. Under normal conditions Tau is known to stabilize microtubules within cells. In AD, however, Tau shows a reduction in its affinity towards microtubules, and this lack of binding to microtubules is thought to be one of the main reasons why Tau aggregates abnormally in this disease. Cantabio’s Tau program aims to stabilize the Tau protein and to preserve its natural function in disease affected cells.
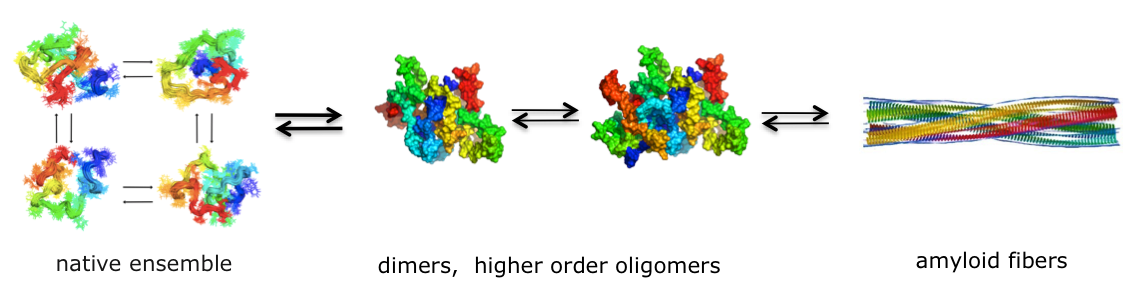
Theoretical representation of the protein (Tau) aggregation process.
